SG Preconception
Genetic screening in order to determine whether someone is a carrier of any of 345 hereditary diseases. For such purpose, we analyse 320 genes and 32,749 causative mutations using targeted massive sequencing.
Establishes whether parents-to-be are carriers of recessive and X-linked hereditary diseases.
Carriers of recessive diseases are normally healthy people but when both parents are carriers of a mutation of the same gene, their children could suffer from the disease. Genetic diseases cannot be cured, but they can be prevented.
SG Preconception is the range of genetic studies from Sistemas Genómicos, which is essential for making decisions regarding different reproduction options, introducing the concept of foresight into reproductive medicine.
The SG Preconception range detects mutations quickly and precisely thanks to Next-Generation Sequencing (NGS), specifically targeted resequencing.
Genetic screening in order to determine whether someone is a carrier of any of 345 hereditary diseases. For such purpose, we analyse 320 genes and 32,749 causative mutations using targeted massive sequencing.
Genetic screening in order to determine whether someone is a carrier of any of 363 hereditary diseases. For such purpose, we analyse 299 genes and 7,400 causative mutations using targeted massive sequencing.
It is aimed at two patient groups:
It is very important to know how the genes on the panel are studied. Specifically, the technique used is Next-Generation Sequencing (NGS). This technique allows us to analyse the sequence of specific genes simultaneously and automatically. In one analysis, we can examine many regions of the genome.
Furthermore, there are diseases that cannot be found using NGS technology but due to a high prevalence rate, they cannot be left off the panel. These diseases are studied in parallel using alternative technology. Specifically, these diseases are: Fragile X syndrome, gene FMR1 (TP-PCR); Friedreich’s ataxia, gene FXN (TP-PCR); Duchenne muscular dystrophy, gene DMD (MLPA) and spinal muscular atrophy, genes SMN1, SMN2 (MLPA).
On this panel we carry out a targeted study. This means that we examine the specific positions of the genome where we know that there are mutations that have previously been described as pathologies. These mutations are described in the HGMD®: Human Gene Mutation Database. All the mutations on the panel have been previously published.
The mutations can be located in different parts of the gene. The outline shows the structure of the human gene with all of its different parts: regulatory elements, flanking regions, exons (DNA coding sequences), introns (non-codifying DNA) and terminator sequences. There are described mutations in all of these regions.
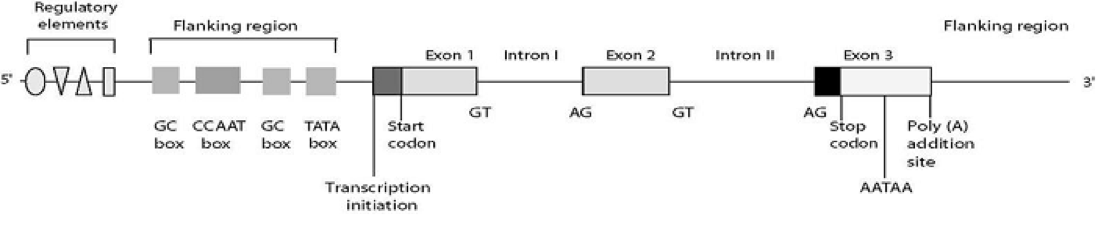
The panel includes mutations described in:
All the mutations found must be confirmed by Sanger sequencing, according to international recommendations. Validation by Sanger sequencing is not included in the study.
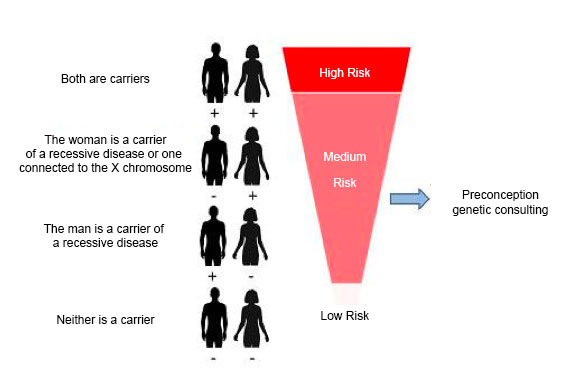
Once the genetic study has been carried out on both members of the couple, the genetic information for both of them is compared for every gene studied and the generic risk is established according to whether or not they are carriers of mutations of the same gene or X chromosome genes in the case of women. The following results can be obtained:
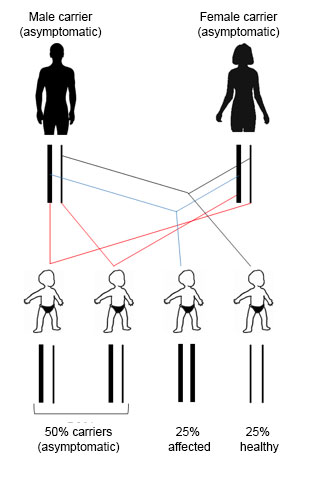
If both members of the couple are carriers of a mutation corresponding to a recessive disease, there is a 25% chance that their children will be affected, a 25% chance of having completely healthy children and a 50% change of having children that are (asymptomatic) carriers.
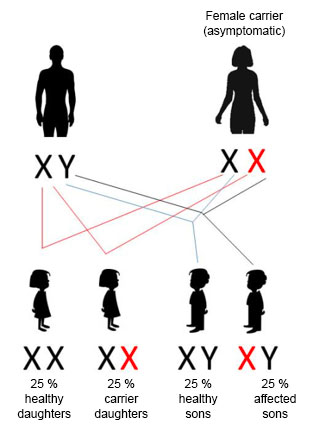
If the woman is a carrier of a mutation connected to an X-linked recessive disease, there is a 25% risk of the children being affected. All those affected will be male.
50% of females will be asymptomatic carriers and 50% will be completely healthy. Regarding male children, 50% will be affected and 50% will be healthy.
A genetic study is carried out on all donors belonging to a gamete donation programme. The genetic information from the donor is included in a database at each centre (held by the centre). When a recipient comes, a Preconception GeneProfile® is carried out. The recipient’s data is cross-referenced with data from the donor bank. If the donor has no mutations that would imply a risk with recipient, they will be deemed an ideal donor. A suitability report will be issued so that the donation can take place.
If the results of the study are negative, the risk of passing on any of the diseases in the panel is completely reduced. However, this does not completely rule out any risk. We have what is known as “residual risk”, which is the risk associated with mutations that cannot be analysed by the technology used in the study or if it does not occur in the sample studied. The residual risk includes:
Sistemas Genómicos S.L.
FinalidadLa finalidad del tratamiento de los datos de carácter personal facilitados a través del presente formulario es la de resolver la consultar que nos plantea acerca de los productos, servicios e iniciativa del responsable. En el caso de que no acepte el tratamiento de sus datos de carácter personal, no podremos atender su consulta.
Adicionalmente, podremos utilizar sus datos de forma anonimizada para realizar un seguimiento estadístico de nuestro servicio de atención al usuario, considerando que contamos con un interés legítimo en la mejora continua de los procesos internos de respuesta.
En el caso de que nos otorgue su consentimiento, sus datos serán tratados para mantenerle informado de las actividades, promociones y novedades empresariales, científicas y formativas
DestinatariosSus datos no serán cedidos a terceros. No obstante, podrán tener acceso a sus datos proveedores de servicios relacionados con el desarrollo y mantenimiento web.
Sus datos podrán ser transferidos a proveedores que nos prestan servicios desde terceros países fuera de la UE acogidos al “Privacy Shield”.
DerechosTiene derecho a ejercitar sus derechos de acceso, rectificación, supresión, oposición, limitación y portabilidad, así como reclamar ante la Agencia Española de Protección Datos (AEPD) en el caso de no estar conforme con el tratamiento de sus datos de carácter personal. Puede encontrar información completa al respecto en la “información adicional”.
Información adicionalLea atentamente la política de privacidad completa aplicable al presente formulario en nuestra política de privacidad.
Sistemas Genómicos S.L.
FinalidadConcertar cita previa para los servicios de atención sanitaria que ofrecemos.
En el caso de que preste su consentimiento, estudiar la documentación clínica que nos facilite de cara a poder asesorarle. En caso de que no consienta el tratamiento de sus datos de salud, no podrá adjuntar documentos.
En el caso de que nos otorgue su consentimiento, sus datos serán tratados para mantenerle informado de las actividades, promociones y novedades empresariales, científicas y formativas
DestinatariosSus datos no serán cedidos a terceros. No obstante, podrán tener acceso a sus datos proveedores que nos prestan servicios, como, por ejemplo, proveedores de servicios médicos, investigadores científicos que colaboran con nosotros o empresas de servicios informáticos.
Sus datos podrán ser transferidos a proveedores que nos prestan servicios desde terceros países fuera de la UE acogidos al “Privacy Shield”.
DerechosTiene derecho a ejercitar sus derechos de acceso, rectificación, supresión, oposición, limitación y portabilidad, así como reclamar ante la Agencia Española de Protección Datos (AEPD) en el caso de no estar conforme con el tratamiento de sus datos de carácter personal. Puede encontrar información completa al respecto en la “información adicional”.
Información adicionalLea atentamente la política de privacidad completa aplicable al presente formulario en nuestra política de privacidad.
Sistemas Genómicos S.L.
FinalidadLa finalidad del tratamiento de los datos de carácter personal facilitados a través del presente formulario es la de resolver la consultar que nos plantea acerca de los productos, servicios e iniciativa del responsable. En el caso de que no acepte el tratamiento de sus datos de carácter personal, no podremos atender su consulta.
Adicionalmente, podremos utilizar sus datos de forma anonimizada para realizar un seguimiento estadístico de nuestro servicio de atención al usuario, considerando que contamos con un interés legítimo en la mejora continua de los procesos internos de respuesta.
En el caso de que nos otorgue su consentimiento, sus datos serán tratados para mantenerle informado de las actividades, promociones y novedades empresariales, científicas y formativas
DestinatariosSus datos no serán cedidos a terceros. No obstante, podrán tener acceso a sus datos proveedores de servicios relacionados con el desarrollo y mantenimiento web.
Sus datos podrán ser transferidos a proveedores que nos prestan servicios desde terceros países fuera de la UE acogidos al “Privacy Shield”.
DerechosTiene derecho a ejercitar sus derechos de acceso, rectificación, supresión, oposición, limitación y portabilidad, así como reclamar ante la Agencia Española de Protección Datos (AEPD) en el caso de no estar conforme con el tratamiento de sus datos de carácter personal. Puede encontrar información completa al respecto en la “información adicional”.
Información adicionalLea atentamente la política de privacidad completa aplicable al presente formulario en nuestra política de privacidad.
Sistemas Genómicos S.L.
FinalidadConcertar cita previa para los servicios de atención sanitaria que ofrecemos.
En el caso de que preste su consentimiento, estudiar la documentación clínica que nos facilite de cara a poder asesorarle. En caso de que no consienta el tratamiento de sus datos de salud, no podrá adjuntar documentos.
En el caso de que nos otorgue su consentimiento, sus datos serán tratados para mantenerle informado de las actividades, promociones y novedades empresariales, científicas y formativas
DestinatariosSus datos no serán cedidos a terceros. No obstante, podrán tener acceso a sus datos proveedores que nos prestan servicios, como, por ejemplo, proveedores de servicios médicos, investigadores científicos que colaboran con nosotros o empresas de servicios informáticos.
Sus datos podrán ser transferidos a proveedores que nos prestan servicios desde terceros países fuera de la UE acogidos al “Privacy Shield”.
DerechosTiene derecho a ejercitar sus derechos de acceso, rectificación, supresión, oposición, limitación y portabilidad, así como reclamar ante la Agencia Española de Protección Datos (AEPD) en el caso de no estar conforme con el tratamiento de sus datos de carácter personal. Puede encontrar información completa al respecto en la “información adicional”.
Información adicionalLea atentamente la política de privacidad completa aplicable al presente formulario en nuestra política de privacidad.
Please, choose an option from below depending on your customer area.
If you do not have this information, contact us on atencion-cliente@sistemasgenomicos.com +34 961 366 150, so we can provide it.
download documentation for servicesPlease, choose an option from below depending on your customer area.
If you do not have this information, contact us on atencion-cliente@sistemasgenomicos.com +34 961 366 150, so we can provide it.
download documentation for servicesRegister your email and we will contact you so you can access